Regulatory Know How
We update our services according to the regulations in force in each market. Since 2011 reporting to regulatory entities.
TRAJECTORY
Our experience and availability mean that all our clients always comply with the times established by government entities
STANDARDS
Experience in implementations in all types of operations Producer, importer / exporter, distributor / 3PL, Hospital / Pharmacy, Parallel importer.
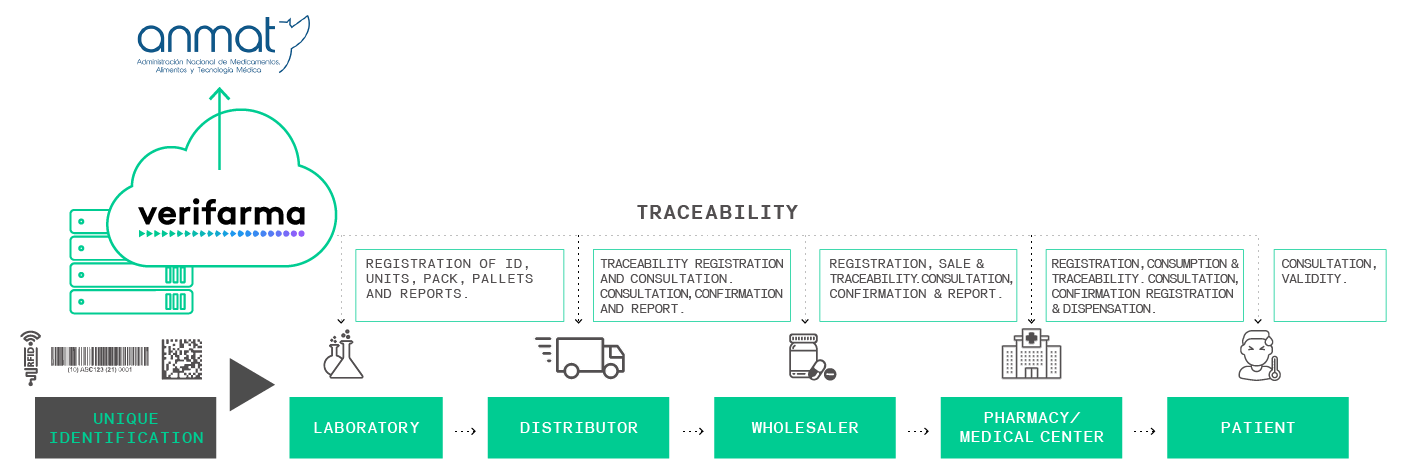
Verifarma is an integral solution allowing the manufacturers of medical devices to administer labels, reports and quality requirements for the compliance with the new regulations on medical devices.
Why Verifarma?
Focus on Regulations
Regulatory know how, we update our services on the basis of the regulations in force in each market.

Quality and Standards
ISO Standards.
Members of GS1 since 2013
24-hours Customer Service
Personalized service, 365 days a year, in Spanish, English and Portuguese.
International solution
With 13 years’ experience and more than 2000 active implementations in 20 countries.
Experience in connections with regulatory entities
ANMAT (Argentina)
SENASA (Argentina)
SEDRONAR (Argentina)
ANVISA (Brazil)
EMVO (Europe)
CRPT (Russia)
SEVEM (Spain)
KOWAL (Poland)
MVO (France)
PAvlina Stisova (Czech Republic)
Supported identification technologies
RFID

CÓDIGO DE BARRAS LINEAL

DATAMATRIX

Contact
España
Carr. Fuencarral 22, Alcobendas, 28108
+34 910 601 552
Argentina
Italia 415, 1°, Vicente López
+54011 5263-9757
Brasil
Avenida Paulista, 2073 Horsa II Cj.1702
+55 11 91307-6193
Ecuador
Teresa de Cepeda N34-260 y Av. de la República
Email: info@verifarma.com
If you are interested in working with us, contact us at info@www.verifarma.com