Regulatory Know How
We update our services according to the regulations in force in each market. Since 2011 reporting to regulatory entities.
TRAJECTORY
Our experience and availability mean that all our clients always comply with the times established by government entities
STANDARDS
Experience in implementations in all types of operations Producer, importer / exporter, distributor / 3PL, Hospital / Pharmacy, Parallel importer.
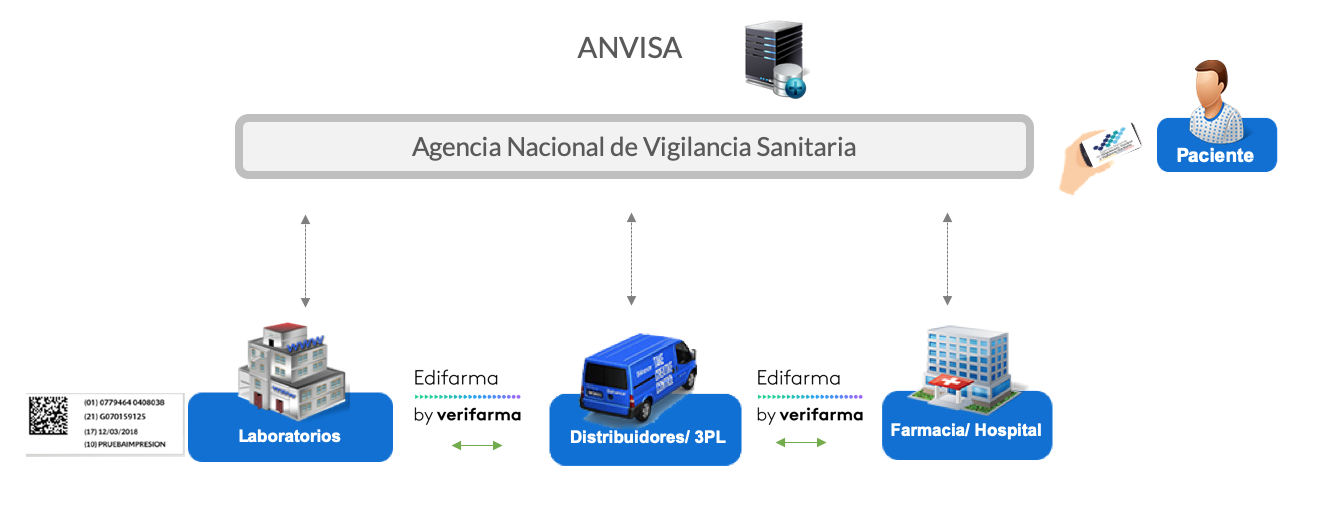
As from its creation in Brazil, in 2014, Verifarma Track & Trace has been focused on offering to the pharmaceutical companies everything needed to comply with the regulations of ANVISA (Brazilian Medicines Control Agency), both, in the consultancy of projects and in the generation of URS and technological solutions.
We provide services of the traceability systems L3, 4 and 5. Among the basic features of our solutions the following can be found:
– Generation and serial number administration
– Administration and assignment of production lines and / or printers
– Integration with CMO and / or MAH for serial administration.
– Horizontal integration with Edifarma, the solution of electronic exchange of data of Verifarma developed and in production since 2014.
– Submission of information to ANVISA with all the requested events.
At present we have production integrations with the main enterprises of market serialization equipment. Having some of them integrated with their L3 and others directly with their production lines. We have also production integrations with 12 enterprises of software L4.
Why Verifarma?
Focus on Regulations
Regulatory know how, we update our services on the basis of the regulations in force in each market.

Quality and Standards
ISO Standards.
Members of GS1 since 2013
24-hours Customer Service
Personalized service, 365 days a year, in Spanish, English and Portuguese.
International solution
With 13 years’ experience and more than 2000 active implementations in 20 countries.
Experience in connections with regulatory entities
ANMAT (Argentina)
SENASA (Argentina)
SEDRONAR (Argentina)
ANVISA (Brazil)
EMVO (Europe)
CRPT (Russia)
SEVEM (Spain)
KOWAL (Poland)
MVO (France)
PAvlina Stisova (Czech Republic)